Chemis-tree Ornaments
by Nicole Ayers, 7th & 8th Grade Math and Science
As part of our study of aqueous solutions and leading into a unit on chemical reactions, students created copper-plated Christmas ornaments. They spent time in art class developing their designs and then brought their work to science class to make their ornaments. Students wrapped pieces of galvanized metal in masking tape and then carefully cut away the areas they wished to expose to chemicals. Concentrated hydrochloric acid was used to remove the zinc layer from the galvanized iron, and then the metal reacted with acidified copper nitrate to deposit a thin layer of copper to the design. The results were quite exciting!


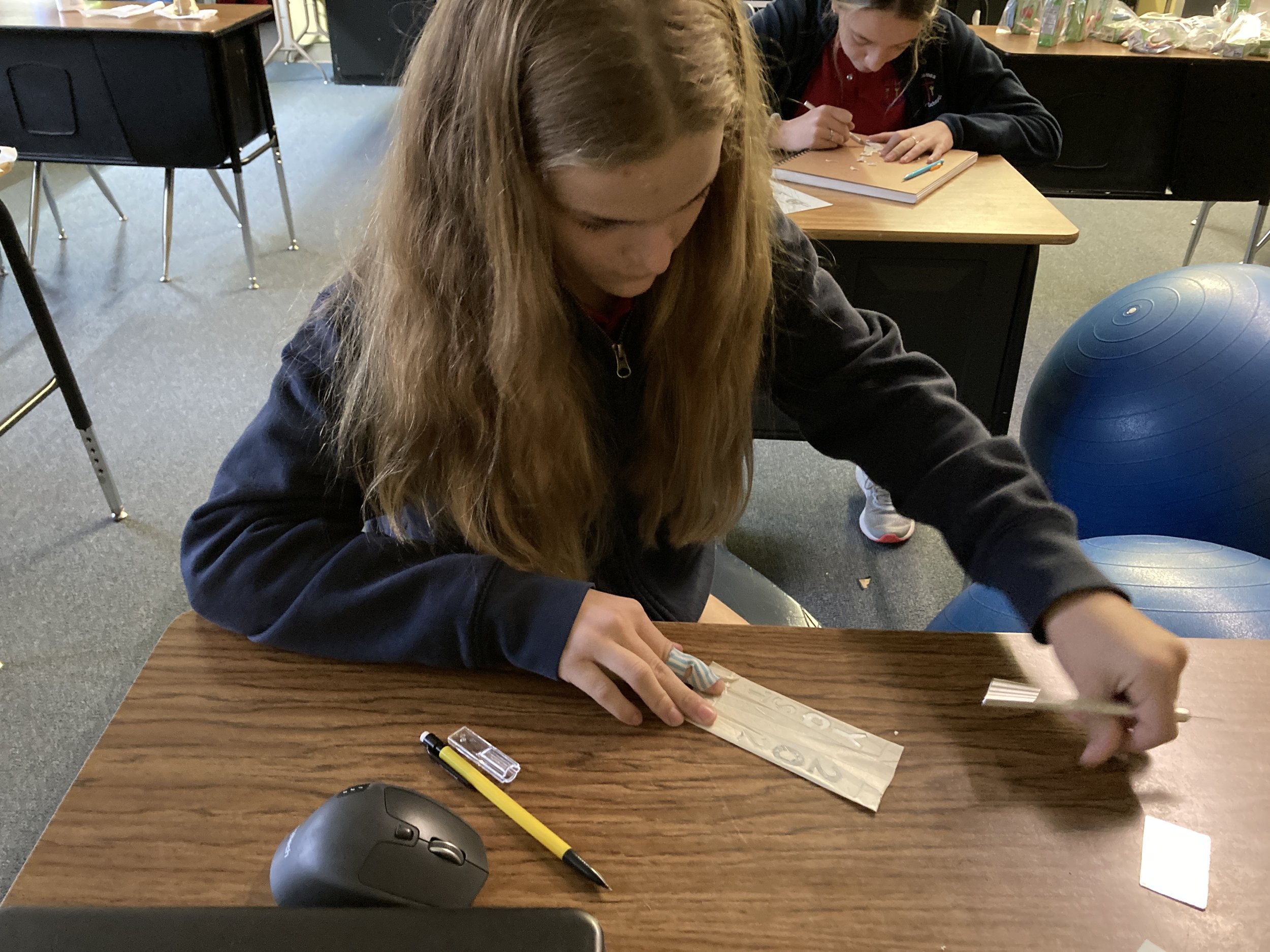



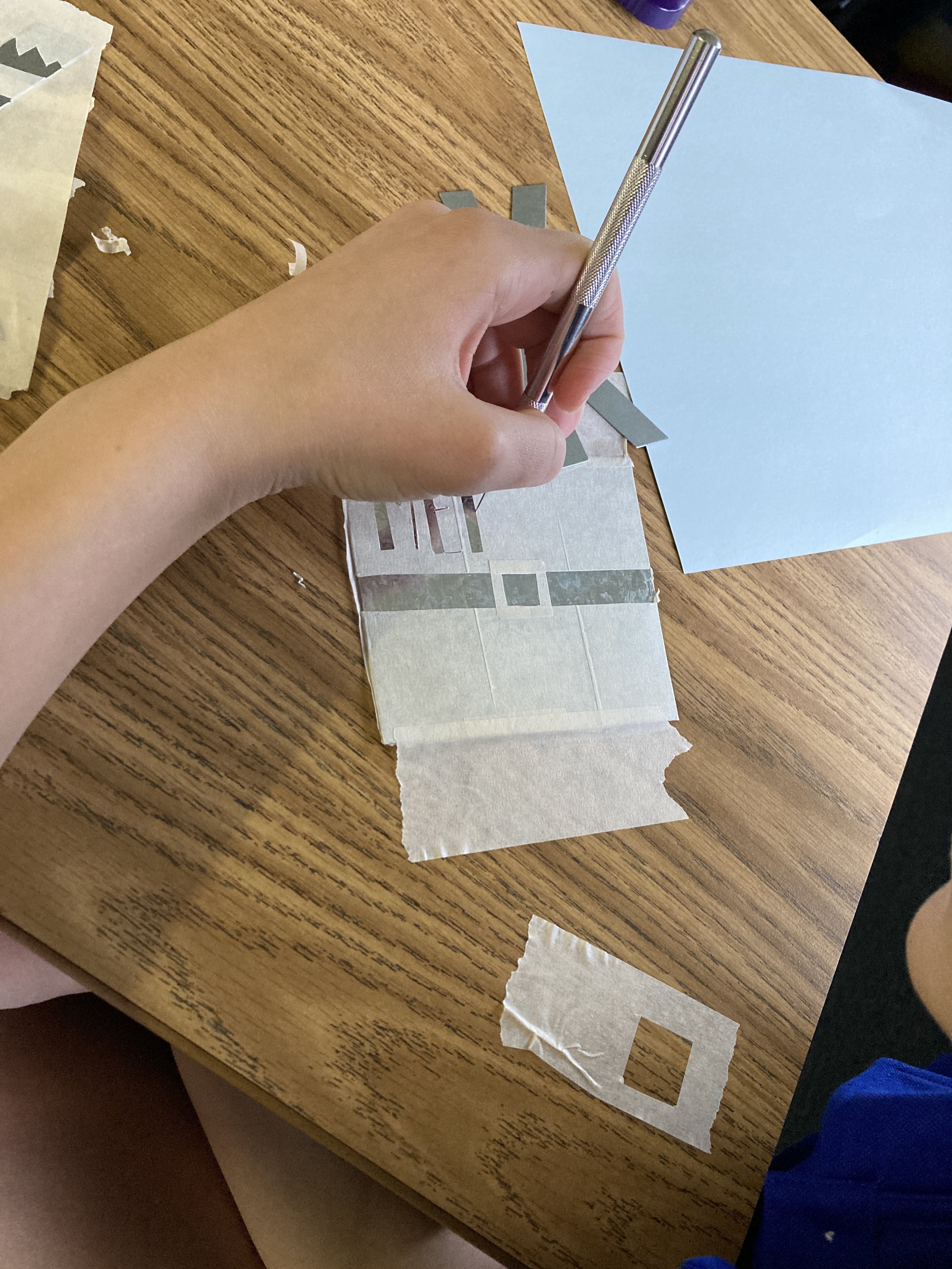


















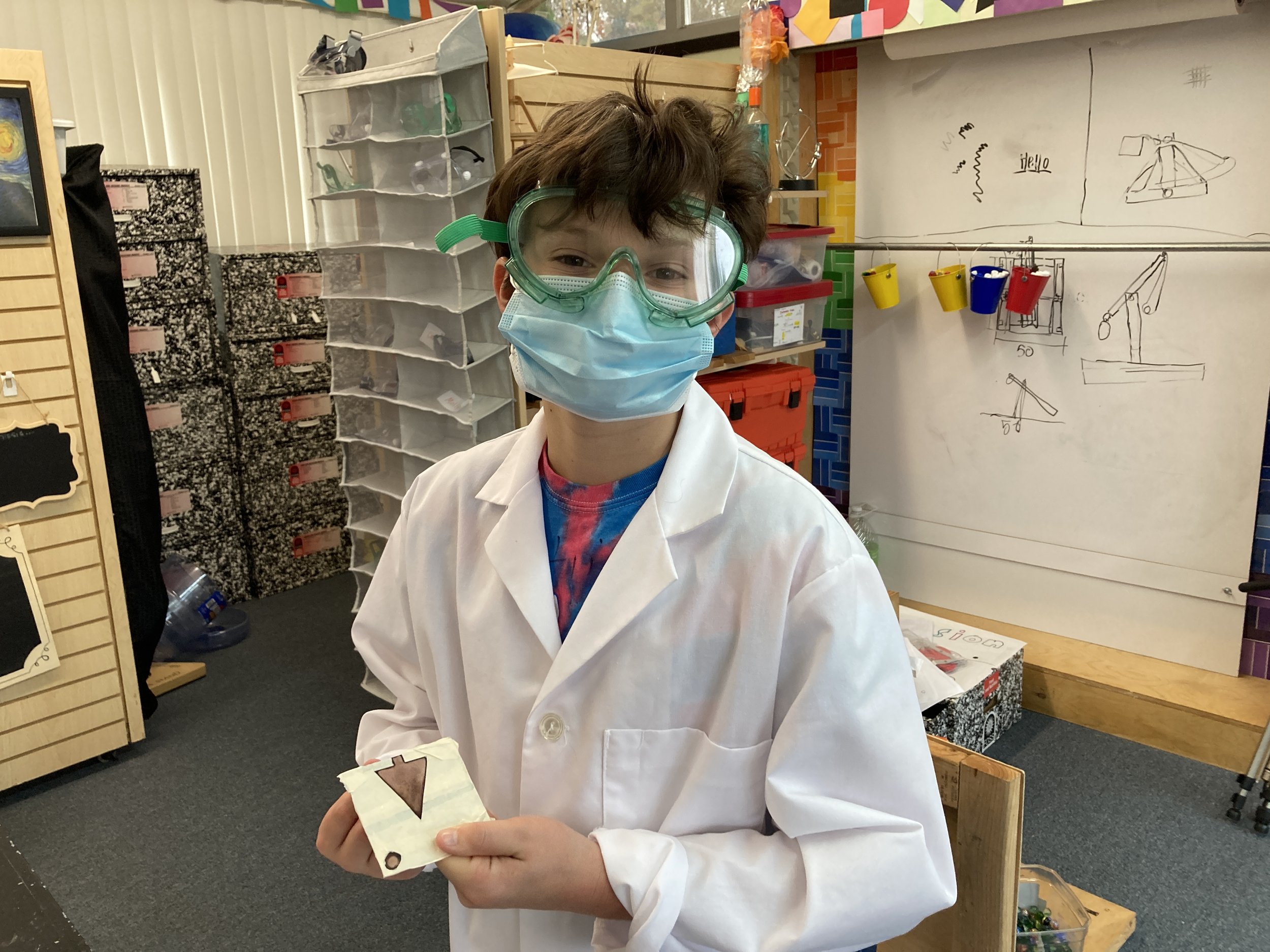










This project was inspired by a blog post from Beals Science: https://www.bealsscience.com/post/2016/12/20/christmas-ornaments-made-with-acid
Printed instructions are available from Flinn Scientific: https://www.flinnsci.com/api/library/Download/174893e5bc714f44abc3aea781e4e9be
If you choose to conduct this procedure on your own, please review the Safety Data Sheet (SDS) for all chemicals involved.
We wish to thank Wright Bros, Inc., for donating squares of cut metal for this project. https://wrightbrotherstxk.com/
